On or bonded to the carbon of an alkene.
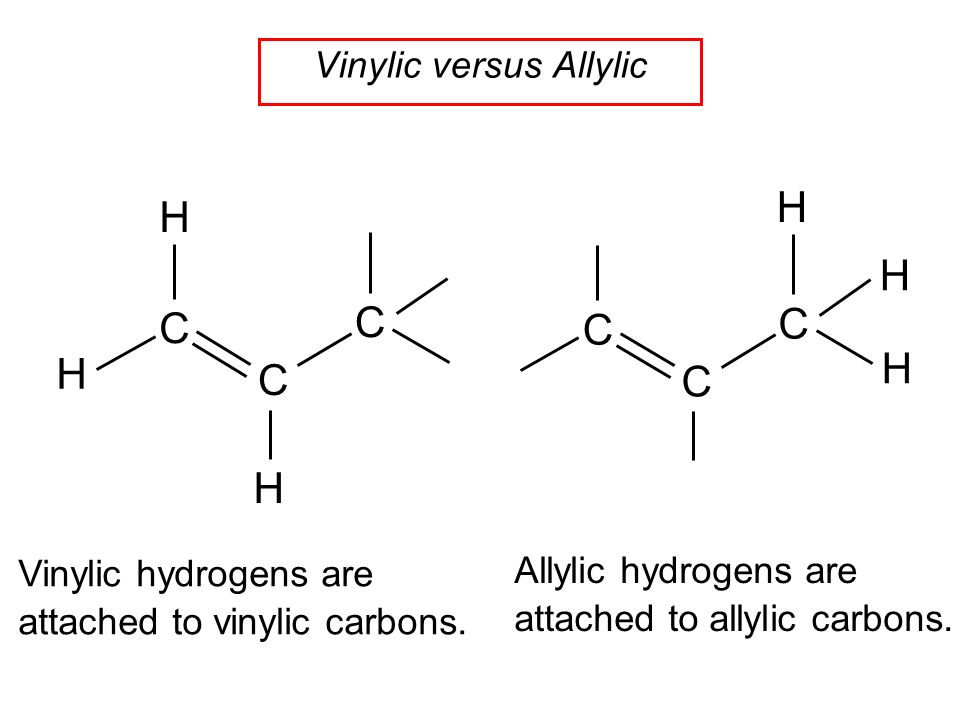
What is allylic and vinylic carbon.
Lewis structure of vinyl chloride a vinyl ic halide.
Vinyl is one of the alkenyl functional groups.
See also allylic hydrogen.
This heightened reactivity has many practical consequences.
Gamini gunawardena from the ochempal site utah valley university back to top.
Key difference allylic vs vinylic carbons functional groups are very important in understanding the different physical and chemical properties of organic molecules the terms allylic and vinyl carbons indicate whether the carbon atom is bonded directly or indirectly to a double bond in a molecule.
This molecule has four vinyl ic positions each marked with.
An allylic carbocation in which an allylic carbon bears the positive charge.
An allylic carbon is a carbon atom bonded to a carbon atom that in turn is doubly bonded to another carbon atom.
See also allylic hydrogen.
Vinylic compounds can produce vinylic polymers such as pvc pvf pvac etc.
Atoms or groups attached to an allylic carbon are termed allylic substituents.
The key difference between allylic and vinylic carbon is that allylic carbon is the carbon.
The organic chemistry tutor 108 416 views 15 37.
Allylic carbon atom can form stable carbocations due to electron delocalization.
Vinylic carbocations are very unstable due to lack of p character.
A styrenic crosslinker with two vinyl groups is called divinyl benzene.
Perbedaan kunci allylic vs vinylic carbons gugus fungsi sangat penting dalam memahami perbedaan sifat fisik dan kimia molekul organik.
On a carbon skeleton sp 2 hybridized carbons or positions are often called vinylic.
An allylic carbon is a carbon atom bonded to a carbon atom that in turn is doubly bonded to another carbon atom.
Istilah karbon alilik dan vinil menunjukkan apakah atom karbon terikat secara langsung atau tidak langsung pada ikatan rangkap dalam suatu molekul.
A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site a group attached at this site is sometimes described as allylic thus ch 2 chch 2 oh has an allylic hydroxyl group allylic c h bonds are about 15 weaker than the c h bonds in ordinary sp 3 carbon centers and are thus more reactive.